First Law of Thermodynamics
Second Law of Thermodynamics and entropy. The First Law is used to categorise the performance of cyclic conversion systems like fossil-fired steam power cycles or geothermal cycles.

Derivation Of The Energy Equation From The First Law Of Thermodynamics Physics Lessons Astronomy Facts Thermodynamics
2 Corollaries of the First Law.

. Free shipping on qualified orders. Q and W are both energies in transit. Is the change in internal energy of the system.
This is the currently selected item. Ad Browse discover thousands of brands. According to the First Law of Thermodynamics when some amount of heat.
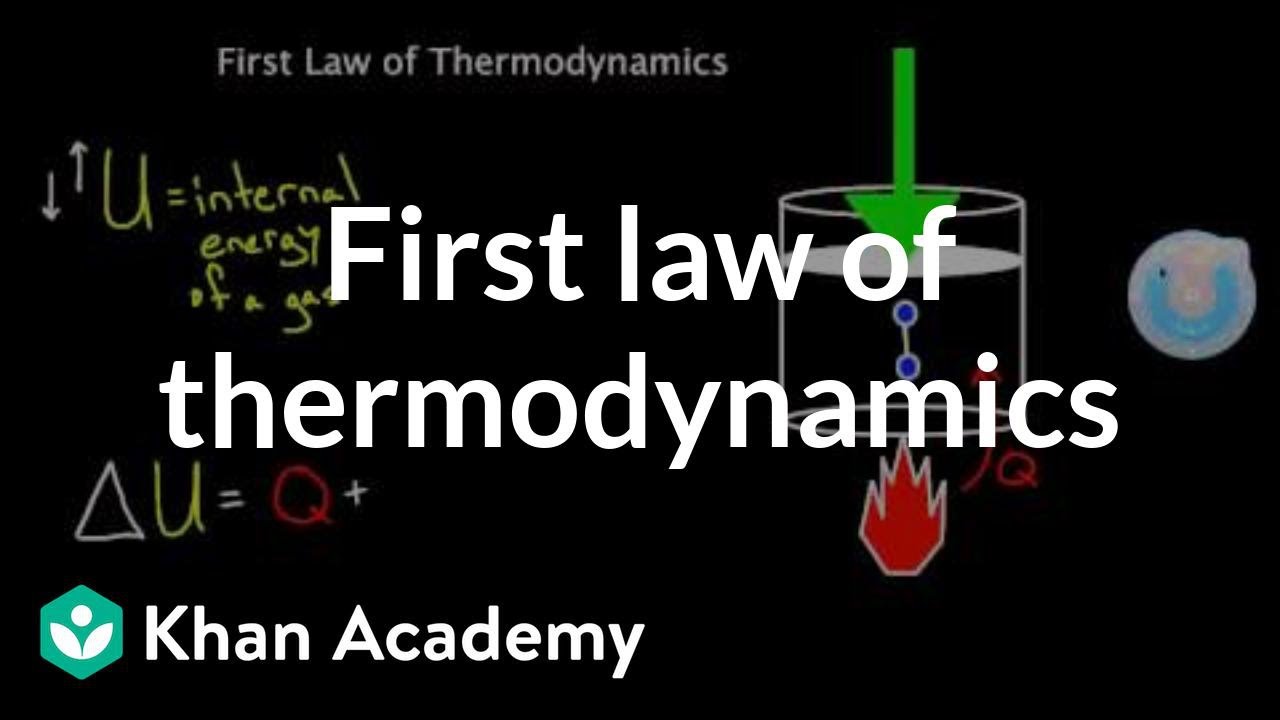
The internal energy of a system can change due to two forms of energy transfer to the system. Q is the amount of heat energy which enters the system. The first law of thermodynamics arguably the most important is an expression of the principle of conservation of energy.
The First Law of Thermodynamics states that energy cannot be created or destroyed. Best PDF Fillable Form Builder. The first law of thermodynamics The laws of thermodynamics are deceptively simple to state but they are far-reaching in their consequences.
In this equation dW is equal to dW pdV and is known as the boundary work. The First Law of Thermodynamics. 4 Specific Heats.
Free easy returns on millions of items. In other words the total energy of the universe remains constant. Theyre moving through the filament which has some resistance and that generates heat.
Energy can be transformed from one form to another but can be neither created nor destroyed. The first law of thermodynamics is given by UQ-W where U is a change in internal energy Q is the sum of all transfers of heat in or out and Wis is the sum of all the work done by or on the system. The first law of thermodynamics is a version of the law of conservation of energy adapted for thermodynamic processes.
Only U represents the capability of being stored. Ad Edit Fill eSign PDF Documents Online. 1 First Law of Thermodynamics.
ATP and reaction coupling. Well I just gave you a hint this thermal energy is due to the electrons moving through the filament. DU dQ dW.
The first law of thermodynamics in terms of enthalpy show us why engineers use the enthalpy in thermodynamic cycles eg. The laws of thermodynamics. The First Law.
The first law asserts that if heat is recognized as a form of energy then the total energy of a system plus its surroundings is conserved. The first law of thermodynamics is a version of the law of conservation of energy adapted for thermodynamic processes distinguishing three kinds of transfer of energy as heat as thermodynamic work and as energy associated with matter transfer and relating them to a function of a bodys state called internal energy. The first law of thermodynamics states that the change in internal energy of a system is equal to the difference between the heat added to the system and the work done by the system.
The classical form of the law is the following equation. The first law of thermodynamics is no more than a statement of conservation of energy applied to thermodynamic systems. 2 Quasi-Static Expansion of a Gas.
Read customer reviews find best sellers. 3 Example Applications of the First Law. 3 Transient filling of a tank.
It can only be converted from one form to another. 1 Adiabatic steady throttling of a gas. Reaction coupling to create glucose-6-phosphate.
First Law of Thermodynamics introduction. This law is usually derived by considering the principle of conservation of energy applied to a system at rest. Internal energy being the sum of the microscopic kinetic and potential energy of all the atomsmolecules in the system.
Again first law of thermodynamics it tells us its not just being created out of thin air it must be converted or being transferred from some place. Brayton cycle or Rankine cycle. 4 The First Law in Terms of Enthalpy.
In general the conservation law states that the total energy of an isolated system is constant.
First Law Of Thermodynamics Thermodynamics Second Law Of Thermodynamics Kinetic Theory

The First Law Of Thermodynamics Internal Energy Heat And Work Youtube Thermodynamics Internal Energy Chemistry

The First Law Of Thermodynamics Jemma Simmons 600px 1 026px Fanedit Quotes Nerd Quotes Fitz And Simmons Thermodynamics

First Law Of Thermodynamics In 2022 Thermodynamics Second Law Of Thermodynamics Internal Energy

First Law Of Thermodynamics Video Thermodynamics Internal Energy Educational Websites

First Law Of Thermodynamics Basic Introduction Internal Energy Heat And Work Chemistry Youtube Thermodynamics Internal Energy Chemistry
Comments
Post a Comment